Although human norovirus (HuNoV) infects millions of people every year worldwide, a lot of questions about the virus still remain unanswered. Its cellular receptor or the spread throughout the human host are just two examples of topics that are still unclear. The main reason for this knowledge gap is an historic lack of (in vivo) models. To fill this gap, we went looking beyond the classic small animal models such as mice, rats or hamsters, and went to the even smaller zebrafish larvae to establish a new, promising model for HuNoV, this was published in 2019 (1).
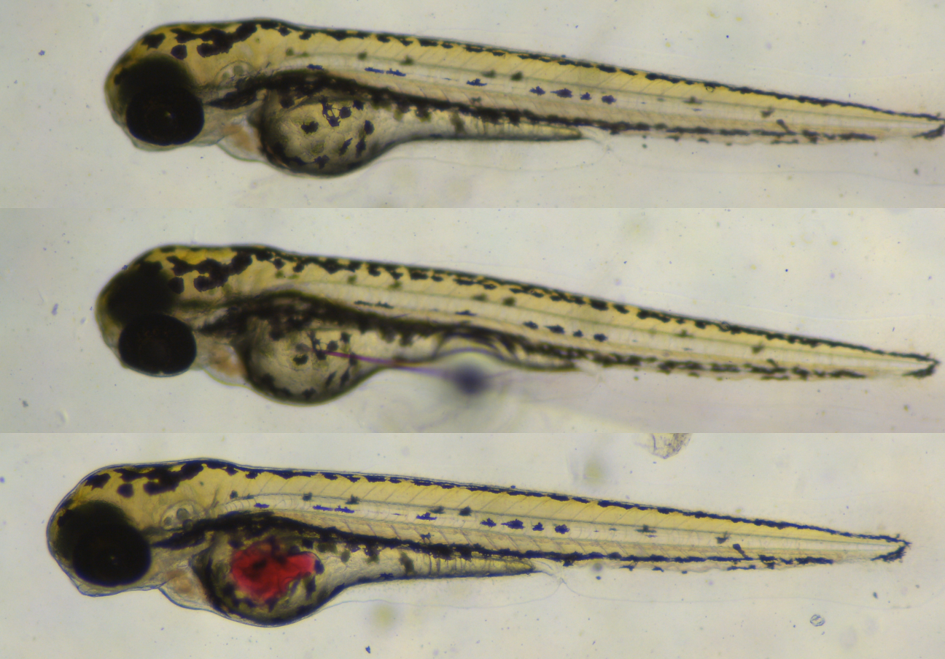
Zebrafish have been a rising star as a model for human viruses in the past few years thanks to their relatively high genetic homology with humans (70%, and even 84% for disease causing genes). Of particular interest to HuNoV is also the morphological similarity of their intestinal system with humans and their optical transparency, making it easy to perform whole-mount staining or live imaging.

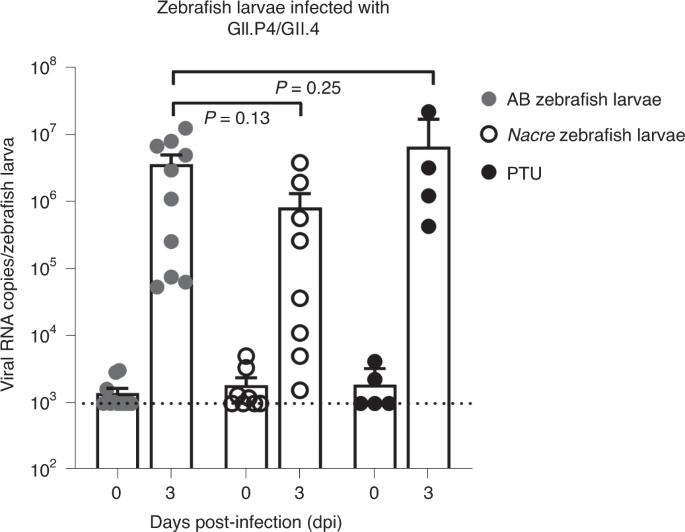
In our original research paper we observed robust viral replication for various clinically relevant HuNoV genotypes (GII.4, GII.6, GI.7,…) and we could inhibit this replication by treating the larvae with 2’CMC, a compound with known antiviral activity against noroviruses. The aim of this protocol we wrote is thus two-fold (i) describing how to house, infect, and monitor HuNoV-infected zebrafish larvae in a BSL2 environment and (ii) how to use this model as a first in vivo step to test new potential antivirals against HuNoV.

The small size, rapid development and high fecundity of zebrafish larvae make it possible to perform medium-to-high throughput compound testing with a possible read-out only 3 days after the start of the experiment. When the larvae hatch 3 days post fertilization most of their organs are already formed and functional, and their yolk, which we use to mimic the faeco-oral transmission route of HuNoV, is prominently present. Our experience tells us that having a well-ordered work-plan is essential when working with (sedated) larvae and virus, and this publication in Nature Protocols will help new adopters in their preparation. Although all individual steps are not difficult on their own, getting to work smoothly with the micro-injector and fragile capillary needle could prompt a technical frustration in the beginning, but as in any situation: practice makes perfect.
In conclusion, we were able to bring forth a game changer to the human norovirus field by providing the first easy-to-work-with in vivo model for HuNoV. We believe that this model will open the doors to many new answers about human norovirus such as the receptor, spread within host or new antivirals. This also the reason we wrote this protocol: to help provide every research team out there with the tools and knowledge to perform their own (viral) infection experiments with zebrafish larvae, not only for HuNoV but, provided some small adjustments to the protocol, potentially for other (human) viruses as well.
References:
(1) Van Dycke J. et al. A robust human norovirus replication model in zebrafish larvae. PLoS Pathog. doi: 10.1371/journal.ppat.1008009 (2019)





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in