Written by Xuexiang Han, Yinlong Zhang and Guangjun Nie
In 1966, a science fiction movie “Fantastic Voyage” described a classic scene where a team of scientists and doctors miniaturized themselves and took a miniaturized submarine to inspect the whole body in order to perform life-saving surgery after they were injected into the blood stream of a dying man. This fascinating imagination has driven numerous scientists to dive into the voyage of the biomedical research, particularly for cancer therapy. 20 years later, scientists discovered that tumor vessels were fenestrated and tumor lymphatic drainage was impaired, leading to selective trap of macromolecules in tumors of rodents1. This so-called enhanced permeability and retention (EPR) effect offers drug-loaded nanoparticles a chance to sneak into tumor tissue and hunt down malignant cells, which echoes with the movie.
However, in clinical practice, the tumors from patients are far more inaccessible and heterogeneous, and nanomedicines relying on EPR effect have so far brought limited benefit for cancer patients2. Tumor cells are minorities, who seed in the “soil” of tumor microenvironment (TME) composed of abundant stromal cells and non-cellular components. The TME not only supports tumor development and metastasis, but also prevents therapeutic drugs from reaching tumor cells3.
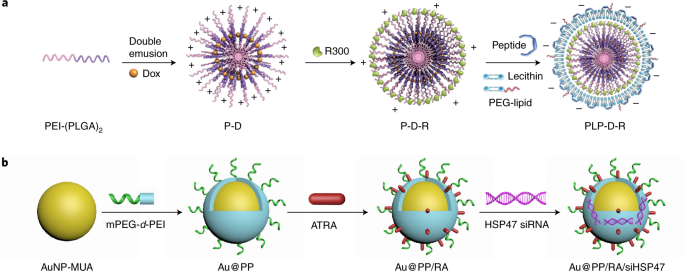
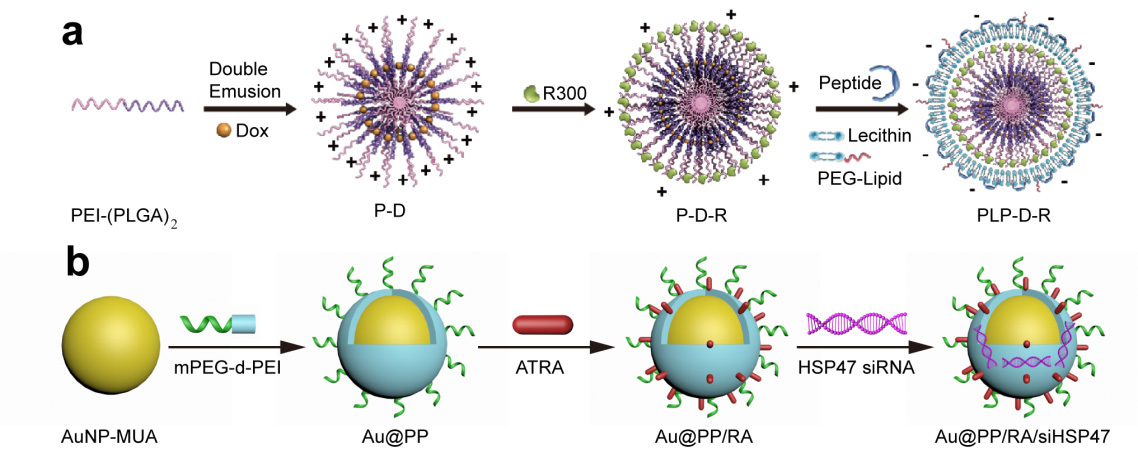
We started to work on targeting and regulating of tumor TME with various nanomedicines 10 years ago, with a specific intention to augment EPR effect and increase the accessibility of tumor-killing drugs4. Since vascular and stromal barriers play crucial roles in blocking drug access to tumor cells, we mainly focus on modulating tumor vasculature and fibroblasts. Tumor-associated platelets (TAPs) are important players in maintaining the integrity of tumor vasculature and cancer-associated fibroblasts (CAFs) are key mediators for extracellular matrix production. Thus, we design two TME-responsive nanomedicines, PLP-D-R and Au@PP/RA/siHSP47, to deplete TAPs and induce CAFs quiescence, respectively (Figure 1)5,6. Our results show that both strategies can significantly improve the tumor permeability and enhance the therapeutic efficacy of forthcoming chemodrugs.

Figure 1. Schematic illustration of PLP-D-R (a) and Au@PP/RA/siHSP47 (b).
In order to back up our conclusion, we realize solid evidence which mainly involves quantitative data is highly appreciated. That is why we use multiple probes (e.g., Evans blue, Nile red and 64Cu-NOTA) and analytical techniques (e.g., fluorescence imaging, PET imaging, electron microscopy and western blot) to confirm the superiority of our strategies.
Currently, manipulating the vascular and stromal barriers to improve tumor permeability is believed to be a promising adjuvant strategy to boost mainstream therapeutic modalities, which attracts a growing interest among tumor biologists. Therefore, we anticipate our approaches to targeting TAPs and CAFs will set examples for the research community to develop TME-regulating nanomedicines to achieve synergistic or additive anti-tumor activity.
References
- Matsumura, Y., & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research 46, 6387-6392 (1986).
- Shi, J., Kantoff, P. W., Wooster, R., & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20 (2017).
- Han, X., Xu, Y., Geranpayehvaghei, M., Anderson, G. J., Li, Y., & Nie, G. Emerging nanomedicines for anti-stromal therapy against desmoplastic tumors. Biomaterials, 232, 119745 (2020).
- Ji, T., Zhao, Y., Ding, Y. & Nie, G. Using Functional Nanomaterials to Target and Regulate the Tumor Microenvironment: Diagnostic and Therapeutic Applications. Adv. Mater. 25, 3508-3525 (2013).
- Li, S. et al. Nanoparticle-mediated local depletion of tumour-associated platelets disrupts vascular barriers and augments drug accumulation in tumours. Nat. Biomed. Eng. 1, 667-679 (2017).
- Han, X. et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun. 9, 3390 (2018)





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in