The 3D brain organoids generally suffer from the lack of a functional vascular network. The neural cells and endothelial cells arise from developmentally distinct lineages, neural cells from neuroectoderm and endothelial cells from mesoderm lineage. Thus, differentiating human ESCs (hESCs) simultaneously into human cortical organoids (hCOs) and endothelial cells are challenging. In order to develop hCOs with vascular system, we initially attempted to co-culture hCOs with terminally differentiated endothelial cells (e.g. HUVEC). This method led to a limited, and short-lived incorporation of endothelial cells into neural tissue (Figure 1). Furthermore, we cultured hCOs on a milli-fluidic chip as described for kidney organoids 1. hCOs on milli-fluidic chip showed an improvement in the number of endothelial cells with tubular structure, which nonetheless did not last long. Culturing organoids in the neural media devoid of growth factors (i.e., VEGF-A) for endothelial cells could be most likely reason of the inability of long-term maintenance of the vascular networks. We, therefore, hypothesized that over-expression of a transcription factor, ETS variant 2 (ETV2) could form vascular structures within hCOs without adding any necessary growth factors.

Figure 1. Immunostaining of whole-mount hCOs with co-culturing HUVEC cells and vhCOs for CD31.
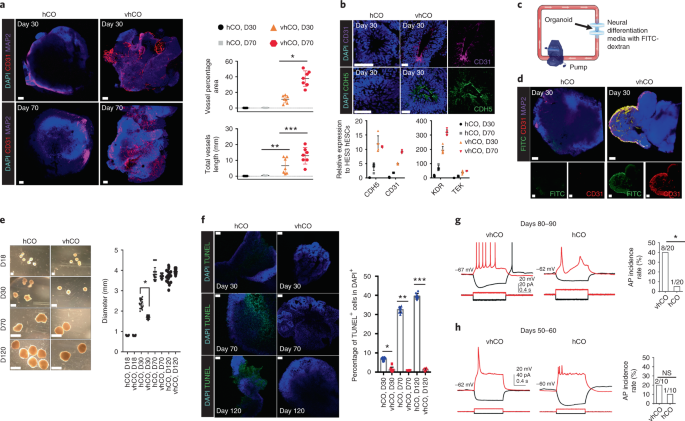
We generated and used hESC line containing the expression of mCherry to identify the cells expressing ETV2. We observed the incorporation of ETV2-induced CD31+ vessel networks in hCOs, and named them as vhCOs (vascularized hCOs). Strikingly, neurons from vhCOs demonstrate the dramatically elevated action potentials (APs) and resembled in vivo neurons corresponding to the later stage in development compared to neurons from control hCOs. The enhanced neural maturation via vascularization was further supported by the expression of mature neuronal genes in scRNA-seq. However, our methods still could not control the location or direction of these vascular networks. The ETV2-induced vascular systems seemed oriented by molecular cues from neighboring neural cells. Regardless of the organization of the vascular-like structure in vhCOs, we observed that the number of apoptotic cells and the hypoxic regions in vhCOs were dramatically reduced compared to hCOs. In terms of size of organoids, despite the fact that vhCOs contain little cellular necrotic area inside of them, their growth was comparable as that of control hCOs. The failure in further increase of organoid size is likely due to the limited number of initial neural progenitor cells or due to the diminished functionality of vessel networks at a later stage of organoid growth.
These cortical organoids implemented with a vascular network represent a powerful tool to study how the interplay between neuronal and endothelial cells play a role in normal and pathogenic neurodevelopment and neurodegenerative disorders. vhCOs can be readily utilized to accelerate our understanding of molecular and cellular disease mechanism, because a growing number of findings demonstrated the importance of neural-vascular interaction in neurological disorders. Notably, this approach could be applied to other region-specific brain organoids and even to different tissue organoids.
If you'd like to learn more about these vascularized organoids, please go read our paper!
This post was written by Bilal Cakir and In-Hyun Park.
References:
1 Homan, K. A. et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16, 255-262, doi:10.1038/s41592-019-0325-y (2019).


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in